Structures and chemical reactions of gas phase clusters have been extensively studied for more than three decades by size-selected experiments. However a large number of different structural isomers were found to be present with increasing cluster size. Thus it becomes more important to separate coexisting isomers in order to discuss microscopic properties from the experimental techniques developed in gas phase. We just started such isomer selected experiment combining ion mobility spectrometry with reflectron time-of-flight mass spectrometry. This method enables us to separate different isomers, based on the fact that terminal velocity of an ion in a drift tube filled with He buffer gas is dependent on the geometrical cross section of the ion. We succeeded in the observation of some cluster ions such as carbon, Cn+.
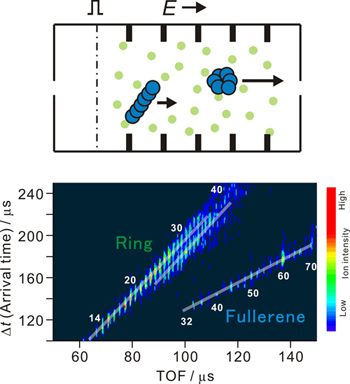
(Figure caption) Top; schematic view of the ion mobility spectrometry. Ions injected from the left run to the right by electrostatic field, colliding with He. Among this process, ion reaches terminal velocity, which depends on the structure (cross section) of the ion. Bottom; Series of cyclic isomers and fullerene isomers of Cn+ cluster ions separated by the present apparatus.
F. Misaizu, N. Hori, H. Tanaka, K. Komatsu, A. Furuya, and K. Ohno
"Isomer-Selected Photoreactions of Gas-Phase Cluster Ions,"
Eur. Phys. J. D 52, 59-62 (2009).
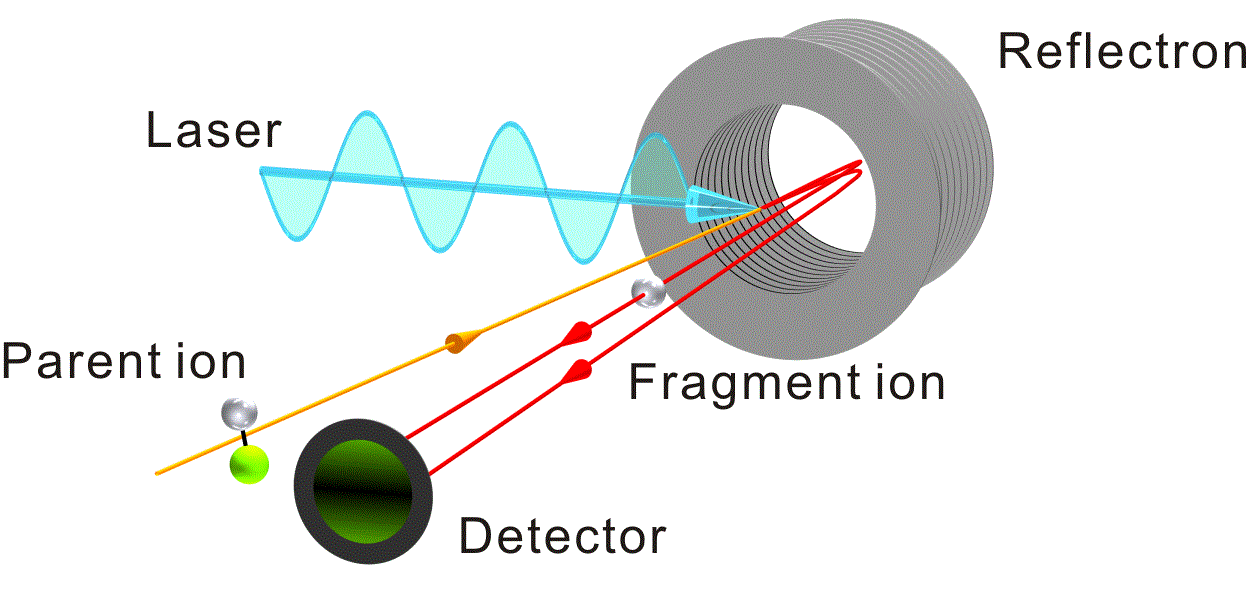 We have developed an apparatus for the imaging detection of mass-analyzed photofragment ions from mass-selected cluster ions using a reflectron TOF mass spectrometer. In this scheme the parent cluster ions, which are mass-separated in the first drift region of the TOF mass spectrometer, are irradiated with a pulsed photolysis laser just in front of the reflection region. The produced photofragment ions are then mass-separated in the second drift region after reflection and are finally detected by an MCP detector with phosphor screen. The fragment-ion images with small recoil energies can be obtained because of the long flight times in the reflectron mass spectrometer. We have adopted this technique for the imaging detection of an Mg+ fragment ion produced upon the ultraviolet laser photolysis of an Mg+-Ar complex.
We have developed an apparatus for the imaging detection of mass-analyzed photofragment ions from mass-selected cluster ions using a reflectron TOF mass spectrometer. In this scheme the parent cluster ions, which are mass-separated in the first drift region of the TOF mass spectrometer, are irradiated with a pulsed photolysis laser just in front of the reflection region. The produced photofragment ions are then mass-separated in the second drift region after reflection and are finally detected by an MCP detector with phosphor screen. The fragment-ion images with small recoil energies can be obtained because of the long flight times in the reflectron mass spectrometer. We have adopted this technique for the imaging detection of an Mg+ fragment ion produced upon the ultraviolet laser photolysis of an Mg+-Ar complex.
H. Hoshino, Y. Yamakita, K. Okutsu, Y. Suzuki, M. Saito, K. Koyasu, K. Ohshimo, and F. Misaizu,
"Photofragment imaging from mass-selected ions using a reflectron mass spectrometer I. Development of an apparatus and application to Mg+-Ar complex", Chem. Phys. Lett. 630, 111-115 (2015).
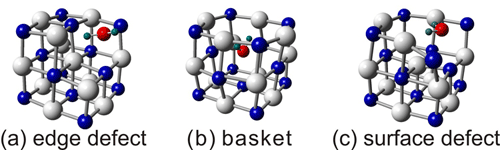 Clusters of ion crystals such as NaI are known to have structures like a model of face centered cubic lattice, and to be stable with rectangular structures. Therefore these clusters are called nanocrystals, in which each particle has an ion form like Na+ or I-. For cluster ions of ion crystals, the ion charge is in general determined by the difference between the number of positive and negative ions. As for singly-charged positive ions of alkali halide clusters, the Na14I13+ ion satisfies the above conditions, which forms a 3×3×3 cubic structure. We have investigated stabilities of nanocrystal ions adsorbed with water molecules by mass spectrometry and theoretical calculation, in order to unveil microscopic picture of initial steps of dissolution or deliquescent processes in salts. In particular we have found that molecular adsorption inside a basket-like deficient structure is important for water adsorption on Na13I12+, which is one NaI smaller than the stable cubic crystal.
Clusters of ion crystals such as NaI are known to have structures like a model of face centered cubic lattice, and to be stable with rectangular structures. Therefore these clusters are called nanocrystals, in which each particle has an ion form like Na+ or I-. For cluster ions of ion crystals, the ion charge is in general determined by the difference between the number of positive and negative ions. As for singly-charged positive ions of alkali halide clusters, the Na14I13+ ion satisfies the above conditions, which forms a 3×3×3 cubic structure. We have investigated stabilities of nanocrystal ions adsorbed with water molecules by mass spectrometry and theoretical calculation, in order to unveil microscopic picture of initial steps of dissolution or deliquescent processes in salts. In particular we have found that molecular adsorption inside a basket-like deficient structure is important for water adsorption on Na13I12+, which is one NaI smaller than the stable cubic crystal.
(Figure caption) Three deficient structures of Na13I12+ adsorbed with water molecules. We found that a water molecule preferentially adsorbed inside the basket structure.
When a metastable helium atom with high excitation energy approaches and collides with a molecule, a collisional reaction (chemi-ionization) occurs. Observing the electron emitted in this process, by changing the collision speed for two bodies, a helium atom can approach a target molecule, it can collide, and the information about the potential energy surface of movement which causes the reaction and leaves can be acquired. We develop world's first two-dimensional electron spectroscopy, and are promoting research of the collisional reaction dynamics of a molecule and an atom about the gaseous phase molecule and the surface adsorbed molecule.
Moreover, the surface reaction process of an adsorbed molecule is observed by two-dimensional electron spectroscopy, and it aims at discovering and solving the unknown chemical reaction in low-temperature conditions.
N. Kishimoto and K. Ohno,Int. Rev. Phys. Chem., 26, 93-138(2007).
Since the effect of electronic correlation movement is strong in the case of a molecule with many electrons which contains a metal atom, it is difficult to investigate about the molecular orbital of an outer shell which is important for chemical reactions, such as an energy level of HOMO (the highest occupied molecular orbital). We have succeeded in the determination of the electronic structure of a complicated molecule with the large electron correlation effect, observing the collisional process in the chemical ionization reaction induced by the metastable excited helium atom with two-dimensional electron spectroscopy, and combining with the result of highly precise quantum chemistry calculation.
Moreover, dissociation of the halogen atom from halogenomethanes is observed by collisional ionization electron spectroscopy, and the collisional reaction process related to destruction of an ozone layer etc. is studied.
N. Kishimoto and K. Ohno,J. Phys. Chem. A, 113, 521-526(2009).
Raising the collision speed for two bodies at the chemi-ionization reaction of the molecule using a metastable excited helium atom will shorten the maximum proximity distance, and the surrounding reaction domain of a molecule approaches the center of a molecule gradually. Since the reaction probability in this case is reflecting spatial distribution of a molecular orbital, it can carry out the probe of the spatial distribution of a molecular orbital from the collision energy dependence of reaction probability. We are studying spatial distribution of two or more molecular orbitals which can be observed at the chemi-ionization reaction of a molecule using a metastable excited atomic probe.
We developed the low-temperature excited atomic beam, made further full use of the three-dimensional decomposition collision ionization electron spectroscopy of collision energy / electron energy / electron emission angle, and have reclaimed a new area of investigation.
M. Yamazaki, T. Horio, N. Kishimoto, and K. Ohno,Phys. Rev. A, 75, 03272(2007).